Abstract
Introduction Intratumor heterogeneity (ITH) is increasingly recognized as an important factor in the variability of B cell non-Hodgkin lymphoma (B-NHL) patient outcomes. It is well established that distinct nodal B cell non-Hodgkin lymphoma (NBNHL) entities originate from different stages of the humoral affinity maturation process, but drivers of ITH are not well understood. In this study, we aimed to investigate whether the maturation process is a driver of ITH.
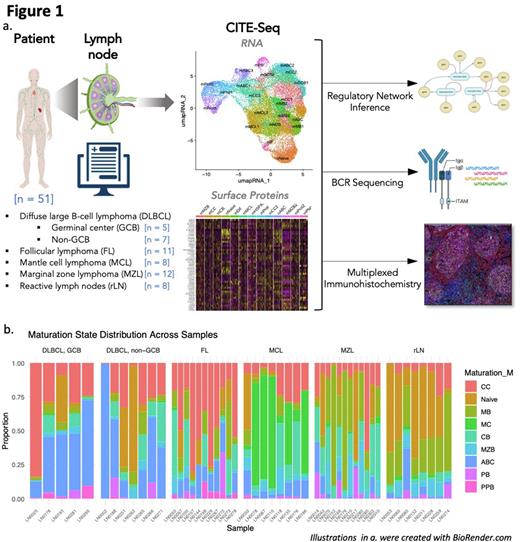
Methods 51 diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), and non-malignant reactive lymph node (rLN) samples from distinct patients were sequenced with CITE-Seq (coupling single-cell RNA-seq with surface epitope profiling), highly multiplexed immunohistochemistry (IHC) with 57 proteins, and single-cell B cell receptor sequencing. Transcription factor activites were inferred from gene expression using the SCENIC package. (Fig 1a)Results Using the rLN samples (n = 8), a single-cell reference map of nodal B cell maturation states was constructed based on known B cell maturation states and their markers. This reference map was used to classify maturation states in malignant samples, which were verified and refined against their maturation marker profiles. Comparable to the non-malignant context in rLN samples, variability in maturation states was also observed for malignant cells in most NBNHL tumors. Although commonalities in composition existed between samples within entities, such as a dominant pre-GC mantle cell population in MCL and abundance of memory B cells in MZL, we observed high variability in maturation state proportions between these samples (Fig 1b). Several GCB and ABC DLBCL samples contained subpopulations of both GC and post-GC phenotypes. BCR-sequencing confirmed that different intra-tumor maturation states observed were monoclonal, and therefore arose from the same cell of origin. Common epigenetic drivers of the maturation processes, such as the transcription factors EZH2 and EGR1, were upregulated in subpopulations advanced in maturation. Known pathways common driving malignant transformation in NBNHL pathogenesis, BCL6 and BCL2, were upregulated in centroblasts and post-GC subpopulations respectively in tumors of the same entities.
Conclusions Our findings reveal maturation is not halted during malignancy, but rather remains a plastic and divergent process. Ongoing maturation and malignant transformation processes are intertwined as the driving forces of intratumor heterogeneity, blurring the known boundaries of NBNHL entities.
Disclosures
Dietrich:Roche: Consultancy; Gilead: Consultancy; Kite: Consultancy; University Hospital Heidelberg: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal